The U.S. Food and Drug Administration (FDA) has approved Lenmeldy (atidarsagene autotemcel), marking a historic milestone as the first gene therapy for children diagnosed with pre-symptomatic late infantile, pre-symptomatic early juvenile, or early symptomatic early juvenile forms of metachromatic leukodystrophy (MLD).
Understanding Metachromatic Leukodystrophy
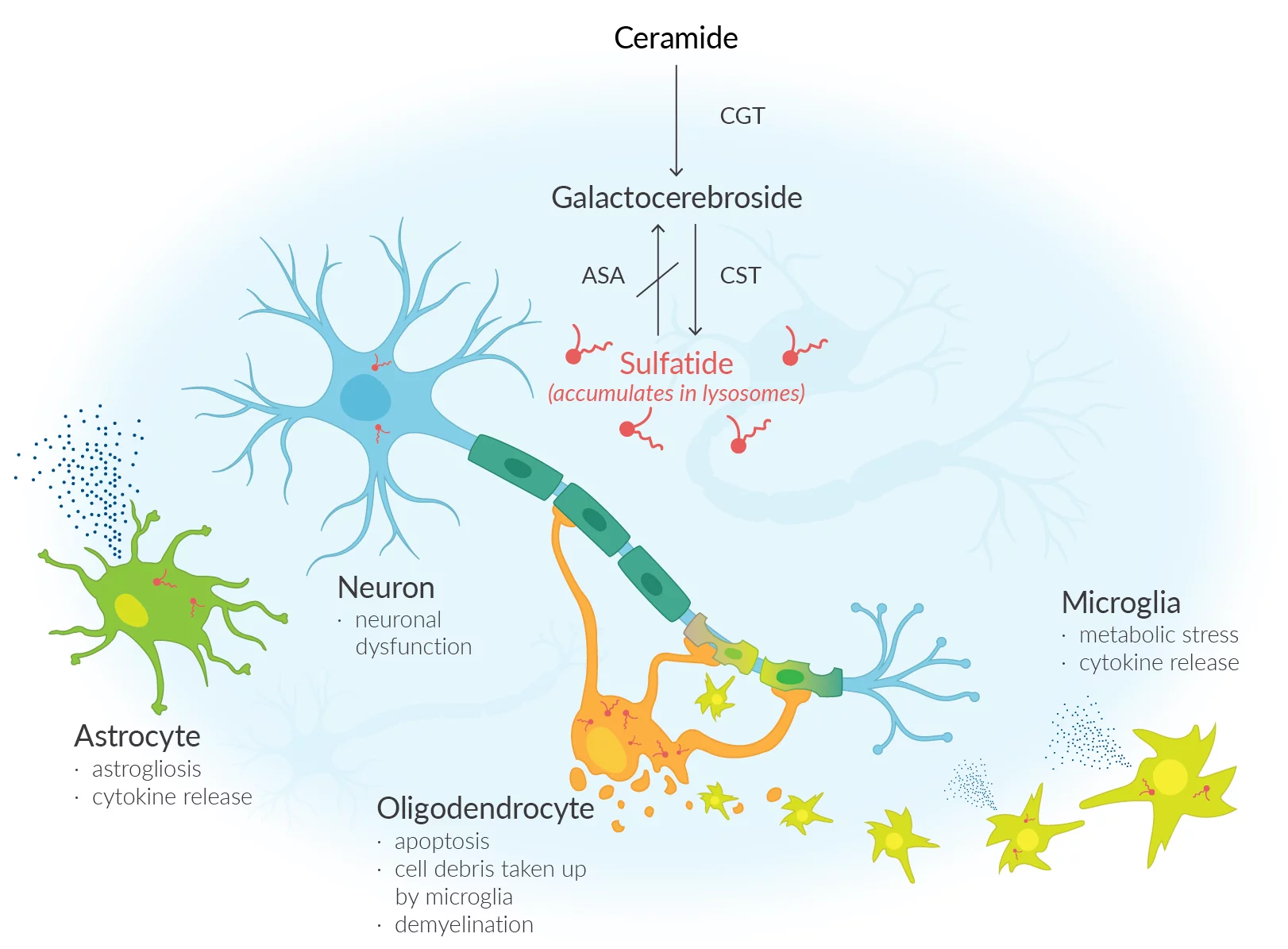
MLD is a rare, debilitating condition caused by the lack of an enzyme known as arylsulfatase A (ARSA). This enzyme deficiency leads to the build-up of sulfatides—fatty substances—in the cells, resulting in damage to both the central and peripheral nervous systems. The outcome is a progressive decline in motor and cognitive function, eventually leading to premature death. MLD affects approximately 1 in every 40,000 individuals in the United States, and until now, no cure has been available. Traditional management has focused solely on supportive care and symptom control.
How Lenmeldy Works
Lenmeldy is a personalised, one-time treatment that uses the patient’s own hematopoietic (blood) stem cells, genetically modified to contain functional copies of the ARSA gene. These modified cells are transplanted back into the patient after high-dose chemotherapy, allowing them to engraft in the bone marrow and produce immune cells capable of generating ARSA enzyme. This process helps break down sulfatides, potentially halting disease progression.
Clinical Trial Outcomes
The safety and efficacy of Lenmeldy were evaluated in two single-arm, open-label clinical trials and an expanded access program involving 37 children. Treated children were compared with an untreated natural history cohort. The primary outcome was the length of time without severe motor impairment—defined as the ability to move and sit without support, or survival.
- At 6 years of age, 100% of treated children were alive versus 58% in the untreated group.
- By age 5, most treated children could walk without assistance.
- Many maintained normal language skills and performance IQ scores—outcomes not seen in untreated MLD cases.
- However, children with early symptomatic forms still showed some decline in motor and/or cognitive function.
Potential Risks and Side Effects
Reported side effects include fever, low white blood cell counts, mouth sores, respiratory infections, rash, gastrointestinal infections, enlarged liver, and medical line infections. Post-treatment monitoring is essential, particularly for neutrophil counts, platelet engraftment, and signs of clot formation or encephalitis. While there is a theoretical risk of blood cancer, no cases have been reported in Lenmeldy-treated patients. Long-term follow-up—up to 15 years—is recommended, including annual blood counts and integration site analysis when indicated.