CHAMPAIGN, Illinois, July 2025 — A pioneering advance in brain imaging technology developed at the University of Illinois Urbana-Champaign promises to revolutionize our understanding of brain function and disease. Scientists have introduced a novel magnetic resonance spectroscopic imaging (MRSI) method that enables high-resolution metabolic imaging of the whole brain in just over 12 minutes—using standard clinical MRI machines.
The study, published in Nature Biomedical Engineering (DOI: 10.1038/s41551-025-01418-4), demonstrates how the new technique can noninvasively detect metabolic activity and neurotransmitter levels across brain regions, as well as identify subtle changes in brain tumors and multiple sclerosis (MS) lesions long before they appear on conventional MRI.
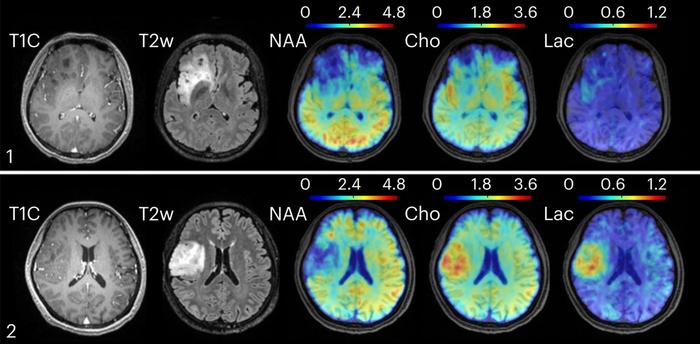 New technology combining high-speed MRI with machine learning methods for data processing found metabolic changes in oligodendroglioma brain tumors. Clinical MRI, in the left two columns, could not distinguish between tumors of grade II, top, and grade III, bottom. However, the new technique found elevated levels of choline and lactate in the grade III tumor [Credit: Yibo Zhao, University of Illinois]
New technology combining high-speed MRI with machine learning methods for data processing found metabolic changes in oligodendroglioma brain tumors. Clinical MRI, in the left two columns, could not distinguish between tumors of grade II, top, and grade III, bottom. However, the new technique found elevated levels of choline and lactate in the grade III tumor [Credit: Yibo Zhao, University of Illinois]
“This innovation adds a new dimension to MRI by allowing us to visualize the brain’s metabolism,” said senior author Professor Zhi-Pei Liang, a leading expert in electrical and computer engineering and member of the Beckman Institute for Advanced Science and Technology. “It fulfills a vision long held by Nobel laureate Paul Lauterbur, one of the inventors of MRI technology.”
Unlike traditional MRI, which captures structural images, or fMRI, which maps blood flow, the new MRSI technique targets molecular signatures—metabolites and neurotransmitters—providing a powerful window into the brain’s biochemical landscape. Postdoctoral researcher Dr. Yibo Zhao, the study’s lead author, explained that “metabolic and physiological changes often precede structural abnormalities, meaning this method can enable earlier diagnosis and intervention.”
The Illinois team overcame long-standing barriers to clinical MRSI—including lengthy scan times and poor signal clarity—by integrating ultrafast data acquisition with physics-informed machine learning algorithms for signal processing. The result: a full-brain metabolic scan completed in just 12.5 minutes, with high spatial resolution and clinical-grade clarity.
The study’s findings are striking. In healthy individuals, the scans revealed diverse patterns of neurotransmitter activity between brain regions. In brain tumor patients, metabolic markers such as elevated choline and lactate differed by tumor grade, even when structural images appeared similar. And in patients with MS, the new method detected neuroinflammatory changes and reduced neuronal activity up to 70 days before they became evident on routine MRI.
Beyond diagnostics, the technique could usher in a new era of precision neurology. “By tracking metabolic shifts over time, we can tailor treatments to individual profiles and monitor their effectiveness more accurately,” said Liang. “This aligns perfectly with the goals of personalized and predictive medicine.”
The project was supported by the Arnold and Mabel Beckman Foundation. As this high-speed metabolic imaging method enters broader clinical trials, its impact may extend across the spectrum of neurological care—from autism and epilepsy to neurodegenerative and metabolic brain disorders.
For more information: Contact Professor Zhi-Pei Liang at z-liang@illinois.edu.
Stay informed at cnke.org for the latest updates in pediatric neuroscience research and innovation.