- Paediatric EEG
- Normal Awake
- Normal Drowsiness, Sleep, Arousal
Introduction 
- The electroencephalograms (EEGs) of infants and children are normally characterized by a greater mixture of waveforms and frequencies than is found in adults.
- The relative predominance of these wave types varies with age.
- There may be considerable intersubject variability, possibly because of differences in maturation.
- Several waveforms, such as the initial response to hyperventilation and posterior slow rhythms of youth, may be normally asymmetrical.
Moreover, infants and young children tend to become drowsy during the recording, and the electrographic alterations with drowsiness are greater than those with adults. These factors create wider limits of normality than might be expected in adults. In addition, the superimposition of two or more waveforms often creates sharply contoured waves that can be mistaken for spikes. Fortunately, most of the clinically significant EEG abnormalities in children are morphologically well defined. However, to identify abnormalities in children's EEGs with confidence, it is first necessary to sharpen one's concept of normal features and their variations.
For each state of alertness (wakefulness, drowsiness, sleep, and arousal), the electroencephalographer interpreting a child's recording should ask the following questions:
- Is the electrical maturation adequate?
- Are there any marked nonartifactual asymmetries beyond those normally accepted for certain waveforms?
- Are there any spikes?
- Is there any excessive focal or diffuse delta activity?
For adults, similar criteria apply:
- Are normal phenomena present: alpha, mu, theta, V waves, spindles?
- Are such features symmetrical or almost so?
- Are states (wakefulness, drowsiness, sleep, and arousal) easy to identify and do they contain normal features?
- Are abnormal spikes present?
- Is there focal or diffuse excess delta activity for state?
Children Awake Recordings Background Activity
Hans Berger was the first to recognize that the frequency of background activity in childhood increases with age, (Gloor, 1969). Studies by Dreyfus-Brisac (1975), Hagne et al. (1973), Pampiglione (1972), Petersen and Eeg-Olofsson (1971), and Samson-Dollfus and Goldberg (1979) have provided the frequency milestones outlined below.
There is no discernible dominant occipital activity until the age of 3 months, at which time a rhythm of 3 to 4 Hz can be seen. Its frequency increases to approximately
- 5 Hz at age 6 months;
- a 6 to 7 Hz rhythm is characteristic from 9 to 18 months.
- By age 2 years, a 7 to 8 Hz rhythm is usual; this increases to 9 Hz by 7 years.
- The mean frequency at age 15 years is 10 Hz.
Background activity can be appreciated best by passive eye closure, as the background can be attenuated by eye opening as early as age 3 months. Gentle passive closure of the eyes can be maintained for a few seconds. A low frequency filter (LFF) of 1 Hz might be helpful to decrease the quantity of movement artifact at such times.
The occipital rhythm may also be seen during crying, as this is often associated with eye closure. In estimating the background frequency, it is important to assure that the patient is not drowsy. Drowsiness should be suspected if there is less than the usual quantity of muscle artifact for age. During drowsiness, the background activity can be 1 to 2 Hz less than that for wakefulness; in children, this can persist for prolonged periods after sleep, even when the child appears alert.
Thus, passive eye closure should be performed at times when complete alertness is assured. There is moderate intersubject variability of background amplitude. The following data will help to assess whether abnormal amplitudes exist, particularly if they are too low, suggesting a focal or diffuse paucity of cortical activity.
Recording with the eyes open, Hagne (1968) found an amplitude of 10 to 20 µV in the first months of life, increasing to 20 to 40 µV at 6 to 12 months. Using the passive-eye-closure technique, Pampiglione (1972) found an amplitude of 50 to 100 µV at age 3 months, increasing to 100 to 200 µV at age 9 months.
During passive eye closure, we have found considerable wave-to-wave amplitude variability in the first year of life, usually from 30 to 100 µV, with occasional waves reaching 200 µV in the latter part of the first year. Pampiglione (1972) reported amplitudes of 50 to 80 µV at age 2 years. The alpha amplitude in the study of Petersen and Eeg-Olofsson (1971) increased to a maximum at 6 to 9 years and then declined. In their study, the average alpha amplitude for children between 3 and 15 years was 56 µV, the amplitude of 90% of their children falling between 30 and 100 µV.
The alpha exceeded 100 µV in 9% and remained between 20 and 30 µV in 1%. All of the latter were 12 to 15 years of age. None of the normal subjects showed a background activity less than 20 µV. Most studies (Corbin & Bickford, 1955; Cornil & Gastaut, 1947) report that alpha activity tends to be higher on the right side. Petersen and Eeg-Olofsson (1971) found alpha-amplitude asymmetries in nearly all children, usually higher on the right. Five percent of their normal population showed an amplitude asymmetry exceeding 20% on the higher side.
They found no relationship between alpha asymmetry and handedness. Posterior Slow Waves and Lambda The alpha rhythm in youth normally and commonly is interrupted by slower rhythms that occasionally combine with the alpha rhythm to create complex and often sharply contoured waveforms. These waveforms appear in the occipital, parietal, and posterior temporal regions. They attenuate with eye opening and may be augmented by hyperventilation. Their prominence may shift from side to side.
Polyphasic Potentials
Polyphasic potentials consist of 250 to 500 ms, medium to high-voltage waves occurring singly or repeating at 2 to 4 Hz. The main body of these waves is usually electropositive. It is often preceded and followed by an alpha wave whose negative-going deflection is greater than usual. Low-amplitude alpha waves may be superimposed upon this 250 to 500 ms potential. These features together create the polyphasic morphology of the phenomenon. Occasionally the accentuated alpha component, together with the after-coming slow wave, can resemble superficially a spike–wave complex, which it is not.
Some polyphasic potentials were found in nearly all of the normal controls reported by Petersen and Eeg-Olofsson (1971), although these potentials were present in only minimal amounts in 30%. The quantity increases gradually during the first decade of life, to peak from ages 9 years to the early teens. This increase in polyphasic potentials may give the false impression of deterioration in sequential EEGs carried out during this age period. Their prominence may be greater at the start of a recording than later.
Polyphasic potentials may be asymmetrical; if so, they are usually of higher voltage on the right. However, the asymmetry should not persistently exceed 50%.
Slow Posterior Rhythm or Posterior Rhythmic Waves
Slow posterior rhythm (SPR) is sinusoidal, of low to medium voltage, 2.5 to 4.5 Hz, and may appear in brief sequences or prolonged runs. Less commonly, it may appear in medium- to high-voltage bursts. This rhythm was originally thought to be associated exclusively with absence attacks, but Petersen and Eeg-Olofsson (1971) found this rhythm in 25% of their normal population. It occurred more commonly in younger children; its incidence increased from 1 year of age to a maximum at 5 to 7 years.
The SPR amplitude was 50 to 100 µV in 90% of the children, but it exceeded 100 µV in 10%. In 25% of the children, the SPR episodes lasted 3 s or more. In 16% of the children, this rhythm occupied more than 10% of the posterior activity; in approximately half, it constituted 2% to 10%. The distinction between this normal phenomenon and a series of rhythmic waves that are actually abortive spike waves is not always clear. Rarely, such rhythmic waves can merge into clearly defined posteriorly situated spike waves; this may occur under the influence of hyperventilation. Thus, if the patient is referred for a question of absence attacks and the SPR is seen, a second attempt at hyperventilation may be indicated.
However, unless clear spike waves are seen, no statement ascribing epileptogenic significance to such waves should be made.
Slow Alpha Variant
This waveform appears to be created by the partial fusion of two alpha waves, creating a notched waveform at half the alpha frequency, as described by Goodwin (1947). Unlike the first two posterior slow waves, this is not peculiar to children. Petersen and Eeg-Olofsson (1971) found such waves in 3.5% of their normal childhood population.
Lambda Waves
Lambda waves are sharply contoured occipital transients evoked by saccadic eye movements scanning a well-illuminated picture or complex design.
The most constant and prominent phase is surface positive, whose duration is 75 to 150 ms; a subsequent 200 to 250 ms negative phase also may occur (Kooi et al., 1978). In early childhood, blinking or other eye movements may evoke sharply contoured occipital transients whose major phase is electronegative; this lasts 200 to 400 ms and may attain 100 to 200 µV. It may be preceded and followed by lower-voltage electropositive phases (Westmoreland & Sharbrough, 1975).
This phenomenon occurs mainly from age 6 months to 10 years, attaining a maximum incidence and prominence between 2 and 3 years of age. Their association with scanning well-illuminated, interesting objects links these transients with lambda waves. Lambda waves may attain higher amplitudes in children, giving them a spike-like configuration. Their occasional asymmetry in normal children furthers this resemblance to occipital spikes. Darkening the room, staring at a blank card, and eye closure will eliminate lambda waves.
Theta and Delta Activity
Varying amounts of diffuse theta activity are seen in the awake records of all pediatric age groups. Theta is seen as a central rhythm at age 3 weeks. The total quantity of theta increases sharply throughout the first years of life, reaches a peak at approximately age 5 to 6 years, and declines thereafter (Hagne, 1968; Corbin & Bickford, 1955). However, as noted in the previous section on posterior slow waves, theta appearing in the posterior head regions may be more prominent at later ages. With the eyes closed or open, theta is the dominant diffuse activity in recordings in the 2- to 5-year-old age group. With eyes closed, its total quantity is approximately equal to that of alpha activity at ages 5 to 6 years, after which alpha very gradually becomes more predominant. However, the relative proportion of alpha and theta varies considerably among normal children. It is often more prominent over the left hemisphere than the right at all ages.
The area of theta distribution is widespread in younger children and tends to be confined to temporal and occipital regions in older children. Despite the aforementioned variations, there is little change in theta frequency with age. Despite its normal prevalence, or perhaps because of it, cerebral disease very rarely manifests itself as focal or diffuse excess theta activity. The rare exceptions are (a) diffuse bursts of 3 to 4 Hz waves not related to drowsiness, which may herald the later appearance of generalized spike–wave discharges, and (b) an awake tracing containing only theta activity in chronic, static, severe encephalopathies. In older children and adolescents as in adults, focal excess theta may be an abnormality. However, its predominance in one region should be consistent before definite clinical significance is ascribed.
Therefore, the electroencephalographer reading the child's EEG does not have to worry about the quantity of diffuse theta activity as long as there is some variability in its quantity and other normal frequencies are present. Perhaps the major reason why the quantity of diffuse or even regionally accentuated theta is difficult to correlate with disease processes is the considerable intersubject variability in theta quantity in all age groups (Petersen & Eeg-Olofsson, 1971; Samson-Dollfus & Goldberg, 1979).
Although frequency analysis indicates that delta activity dominates during the entire first year of life (Hagne et al., 1973; Pond, 1963), delta and theta appear approximately equally prominent to visual inspection in the first year. The absolute quantity of delta activity increases throughout the first year (Hagne, 1968) and actually continues to do so until the fifth year (Corbin & Bickford, 1955). However, this increase is less than that for theta; for this reason, theta activity becomes more prominent as the first year progresses and is the dominant diffuse awake activity in the 2- to 5-year-old age group. Delta activity is commonly diffuse in early childhood but may be transiently asymmetrical, the side of maximum expression shifting over the course of the EEG recording.
Therefore, it is important to examine long stretches of the recording before concluding that delta clearly predominates in one region. Small amounts of diffuse delta activity can normally be identified in older age groups, even into adolescence.
Beta Activity
Petersen and Eeg-Olofsson (1971) found beta activity at 10 to 20 µV in various quantities in all awake recordings of their 743 unmedicated normal children. Amplitudes above 20 µV appeared in only 1%. Other principles concerning beta activity in awake recordings for adults also apply to children.
Central Rhythms
Sustained runs of activity develop earlier in the central regions (C3, C4) than in any other area (Hagne et al., 1973). Pampiglione (1972) found a 6 to 7 Hz rolandic rhythm before 3 months of age whose frequency increases to 8 to 10 Hz after 3 months. Its quantity augments further between 6 and 12 months (Hagne, 1968). Even in the 1 to 5 year-old age group, the most prominent activity while awake with eyes open resides in the central region. Moderate asymmetries of such activity are acceptable. However, a complete and persistent unilateral absence of central rhythms usually reflects a lesion on that side. On occasion, such central rhythms can become even more sharply contoured than the mu they resemble, giving the appearance of spikes.
To assure that such forms are not spikes, look for similar sharply contoured waves that are clearly central or mu rhythms. Unless a definite qualitative distinction can be made, call them both normal central rhythms.
Frontal Activity
During wakefulness, there is normally a relative paucity of activity over the frontal regions in the early years (Hagne, 1968). Subsequently, theta activity predominates. This rarely attains a voltage equal to that in the central regions except in drowsiness.
Asymmetries
In adults, a chronic, mild focal lesion can be detected by careful side-to-side comparisons. However, this is more difficult to accomplish in children because (a) transient asymmetries occur more commonly (especially in the very young) and (b) some types of activity are normally asymmetrical.
Such activities include the following.
- Theta activity is usually more abundant over the left hemisphere (Petersen & Eeg-Olofsson, 1971);
- posterior slow waves often appear maximally on the right, particularly over the right posterior temporal region (T6), as compared with the left (T5) (Aird & Gastaut, 1959).
Central rhythms may be asymmetrical . Hyperventilation may initially cause a greater buildup over one hemisphere, usually the left; this left–right difference is usually seen principally over the temporal regions. In infants, brief runs of delta activity may shift from side to side throughout the recording.
Activation Procedures
Any method that may elicit an EEG abnormality which has not occurred in a routine recording falls into the broad category of an activation procedure.
Hyperventilation
From age 4 years, children can cooperate with hyperventilation; their enthusiasm for the procedure usually is greater than that of adults. The main role of hyperventilation is to elicit generalized spike–wave discharges when they are not present on the “resting” recording.
Less commonly, focal spikes appear. Other focal abnormalities may also be revealed. The “buildup” of slow waves is characteristically greater in children than in adults, particularly in children around 10 to 12 years of age (Petersen & Eeg-Olofsson, 1971). The first effect is usually an accentuation of the dominant frequency in the resting recording (e.g., alpha), followed by augmentation of theta; finally, 3-Hz rhythmic waves appear (Corbin & Bickford, 1955). These slower waves may occur posteriorly before becoming diffuse and maximum anteriorly.
A slightly greater buildup on one side, usually the left, may occur in healthy subjects. Sometimes hyperventilation creates sharply contoured waveforms whose differentiation from true spikes is difficult. This occurs particularly when the resting record contains a rich mixture of waves, such as beta and theta. In this situation, it is better to read the hyperventilation response as normal unless clear identification of spikes can be made. Revealing your uncertainty by terms such as “spikey” may mislead the reader of your report. No pathological significance can be given to the abruptness or amount of buildup or to its persistence after the apparent end of hyperventilation. The quantity of slow waves depends primarily on the degree of hypocapnea produced and the blood sugar level (Kellaway, 1990).
Moreover, many subjects continue to hyperventilate despite the technologist having asked them to stop.
Photic Stimulation
Four types of results occur with photic stimulation: (a) “driving” i.e., response of varying morphology that is time-locked to the flash rate; (b) frontal myoclonic potentials, also time-locked, reflecting myoclonus of periocular and scalp musculature; (c) photoparoxysmal response and (d) no apparent effect.
Sleep Recordings
The reliable interpretation of pediatric and adult EEGs requires a thorough appreciation of drowsy, sleep, and arousal phenomena, as patterns occur in these states that do not appear in normal subjects. In addition, the morphology of common sleep patterns may differ between children and adults. Lack of a clear correlation between clinical and EEG manifestations of state reflects a diffuse encephalopathy in children and adults.
Drowsiness
Electroencephalographic signs of drowsiness commonly appear before the child, particularly an infant or younger child, appears drowsy to the technologist.
A drowsy child's eyes may be wide open, and he or she may be restless. The technologist should record the entire process of falling asleep so that the electroencephalographer can interpret the many changes that occur. A child may drop quickly from light drowsiness to deep sleep; intermittent recording would miss lighter sleep stages. It is common for young children to fluctuate fairly rapidly between drowsiness, sleep, and arousal. Continuous recording will assure that an abnormality that occurs only once or during one state of alertness or sleep will not be missed. The most common drowsy pattern in children is diffuse rhythmic to sinusoidal theta, which gradually replaces the awake pattern and may persist for several minutes.
This may be maximal centrally, more posteriorly, or frontocentrally. It is present from age 3 months to 4 years and then declines in prominence, to be only minimally evident after 6 to 7 years (Dale & Busse, 1951; Kellaway & Fox, 1952; Brandt & Brandt, 1955). The frequency is 3 to 5 Hz in the first year of life, increasing gradually to 4 to 6 Hz by age 4 to 5 years. Its amplitude is variable, up to 200 µV. Delta may augment slightly in drowsiness, but less so than theta. Less common but more dramatic are generalized bisynchronous bursts of 2 to 5 Hz, rhythmic to sinusoidal, high-voltage waves that may exceed 350 µV (Kellaway & Fox, 1952). They are usually maximal frontocentrally.
They first appear at 14 to 18 months and are most common between the ages of 3 and 5 years. However, they may be a component of drowsiness and light sleep to age 11 years. The frequency of such waves gradually increases with age. Because of the superimposition of some background activity upon such bursts, they may be confused with generalized spike waves, which may also appear preferentially with drowsiness. Identification of a burst as a spike–wave complex should be reserved for instances where the spike is clearly distinct from background activity. These normal bursts disappear in moderate sleep, unlike spike–wave complexes, which usually persist or may become more frequent in sleep.
The continuity of any occipital rhythm of wakefulness may break up in early drowsiness without initial slowing. On other occasions, there is slowing and increasing amplitude of the occipital rhythm; this may then merge with continuous sinusoidal theta rhythm described previously. Indeed, combinations of all these drowsy patterns commonly occur. From age 5 to 6 months, beta activity at 25 Hz occasionally may become more prominent in drowsiness and light sleep, distributed diffusely or with an anterior or a posterior maximum.
Transient and shifting beta asymmetries may normally occur. Beta, which occurs initially at about 5 µV, increases to a maximum of 30 µV at 12 to 18 months, following which there is a gradual decline. Unmedicated children older than 7 years of age show little beta in early sleep (Kellaway & Fox, 1952). Sedative medication will promote the appearance of beta at any age.
Vertex Waves
Rudimentary vertex waves (V waves) appear in light sleep as early as 3 to 4 months of age but usually are well developed by 5 months (Kellaway & Fox, 1952). They achieve their maximum expression at 3 to 4 years, following which there is a modest decline until adolescence, when the adult form is attained. Compared with those in adults, V waves in children are higher in voltage and briefer. Moreover, spontaneous V waves may occur in sequences, a phenomenon seen less often in adults. V waves may be electropositive, electronegative, or diphasic.
They are often followed by a single slow wave that is opposite in polarity to the major deflection. shifting apparent asymmetries of V waves are commonly seen using bipolar anteroposterior or coronal montages. Such apparent asymmetries may be due to field cancellation effects and should be verified using an ipsilateral ear reference run. However, even then, shifting asymmetries may normally be seen. Unfortunately, there is no reliable relationship between the side of the larger or smaller V waves and a unilateral lesion.
Spindles
Rhythmic sequences of 12 to 14 Hz waves appear in light sleep a few days after term (Kellaway & Fox, 1952). These may be the forerunners of spindles. Initially of low voltage and poorly defined, they become more clearly expressed by 3 to 4 months of age. From ages 3 to 9 months, spindles are almost invariably present during the non–rapid eye movement (non-REM) sleep of a normal child. They appear principally over central parietal (C3,4; P3,4) and sagittal (Cz; Pz) regions but may extend frontally (F3,4) and diffusely.
Spindles appear frontal–central in adults. Several measures of spindle quantity indicate that they are most abundant at age 3 to 9 months. The quantity declines to a minimum at 22 to 54 months, after which there is a moderate increase (Lenard, 1970; Schulte & Bell, 1973; Tanguay, 1975). Thus, Tanguay found the maximum mean number of spindles per 10 s to occur at age 4 to 6 months; this decreased to a minimum at 27 to 54 months. Similarly, these authors found the mean length of individual spindles to be 1.5 to 1.8 s at 4 to 6 months, falling to 0.5 to 0.6 s at 25 to 54 months.
Spindle length later increased, reaching 0.9 s. Lenard (1970) found a mean spindle duration of 2.5 s at 3 months and 0.75 s at 22 months. Kellaway and Fox (1952) found spindles as long as 3 to 4 s in very young infants. In contrast, the spindle wave frequency remains relatively consistent at 13 to 14 Hz from infancy to 4 to 5 years (Kellaway & Fox, 1952; Tanguay et al., 1975). After 3 to 5 years, a 10 to 12 Hz frontally dominant spindle occurs in about 5% of normal children (Kellaway, 1990). Its field may extend to the occipital region. Unlike adults and older children, interhemispheric asynchrony of spindles occurs more commonly among subjects less than 1 to 2 years of age (Lenard, 1970; Tanguay et al., 1975).
Although spindles tend to become more synchronous after this age, mild degrees of asynchrony may normally persist throughout the first decade. In addition to having the sinusoidal shape of adult spindles, the spindles of infants and young children may also be comb-shaped. Combined with V waves and other normal central rhythms of sleep, such comb-shaped spindles may create waveforms resembling spikes, which they are not.
Occipital Sharply Contoured Waves and Delta
These high-voltage diphasic occipital waves appear in moderate sleep and increase in incidence as sleep deepens. In deep sleep, such waves are associated with high-voltage, arrhythmic, posteriorly situated delta activity.
Such posterior phenomena are commonly seen in sleep of children less than 5 years of age; their prominence declines thereafter (Slater & Torres, 1979).
14- and 6-per-Second Positive Spikes
“Fourteen and six per second positive spikes” is a 60 to 70 µV comb-shaped phenomenon whose electropositive sharp components repeat at 14 and/or 6 to 7 per second. A burst may last as long as 3 s; rarely, only a single electropositive spike appears, principally in drowsiness or light sleep.
Occurring independently in either hemisphere, the 14 and 6 field is widespread but centered over the posterior temporal regions. Thus, montages with long interelectrode distances, including interhemispheric linkages, best reveal their presence. Much of the earlier work attributing clinical significance to this normal finding appears to be based on the assumption that a spike-containing phenomenon must be abnormal. Unfortunately, most of these earlier studies lacked precise criteria for the selection of normal control subjects.
The following authors found 14 and 6 per-second positive spikes in their control populations: Schwartz and Lombroso (1968) 55%, Metcalf (1963) 26%, Gibbs and Gibbs (1964) 20%, Lombroso et al., (1966) 58%, and Demerdash et al. (1968) 7%. Schwartz and Lombroso (1968), Lombroso, et al. (1966), Reiher & Carmant, 1991 and Eeg-Olofsson (1971) did not find any correlation between the presence of “14 & 6” positive spikes and personality variables or vegetative-like complaints. Most studies (Demerdash et al., 1968; Eeg-Olofsson, 1971; Petersen & Akesson, 1968) found an increased quantity with age of the child, with a peak incidence at early adolescence.
Lombroso et al. (1966) found considerable intersubject variability in the quantity of “14 and 6.” In EEGs of children with acute encephalopathies and a depressed level of consciousness, Drury (1989) identified 14 and 6 per-second positive spikes resembling those of normal sleep in duration, morphology, frequency, and repetition rate, but they differed in being evoked by stimulation.
Arousal
At less than 2 months of age, arousal consists of a simple decrease in voltage of the tracing. At 2 to 3 months, a rudimentary diphasic slow wave may occur in response to stimuli.
The initial diphasic component may be absent if arousal is spontaneous. Resembling a vertex wave, this phenomenon becomes better developed by 5 months; it may merge into a series of delta waves. If further arousal ensues, 4 to 8 Hz rhythmic waves appear diffusely, maximal frontocentrally, lasting 1 to 5 s or longer. First seen at 7 months (Kellaway & Fox, 1952), such waves become well developed at 13 months and remain prominent until age 3 to 4 years. Accompanying or immediately following this rhythmic theta is diffuse 1 to 3 Hz, semirhythmic to arrhythmic diffuse delta. This delta component of arousal may initially be accentuated anteriorly but tends to persist longer posteriorly. Such delta waves may appear simultaneously anteriorly and posteriorly as independent activity.
The posterior delta is usually the slower. Over the next several seconds, the frequency of this rhythm increases to 4 to 5 Hz, when it becomes indistinguishable from a drowsy rhythm. This slower rhythm first appears at 2 to 3 months, is well established at 4 to 5 months, is maximally expressed at 12 to 18 months, and declines after 4 to 5 years. Of course, this sequence may abort at any point if the child returns to sleep.
Drowsiness and Arousal from 7 to 16 Years and in Adulthood
Medium-voltage, 5 to 7 Hz rhythmic waves lasting 5 to 30 s appear anteriorly in drowsiness; the frequency tends to increase with age within this range.
During this period, the arousal gradually attains the adult form. Its rhythmicity–that is, its sinusoidal waveform–distinguishes such theta from abnormal theta bursts that are semirhythmic, reflecting the superimposition of multiple theta frequencies. Except for arousal from deep, non-REM sleep, where a brief delta/theta burst may occur, adolescent and adult arousal should consist of conversion from sleep to awake EEG characteristics within 1 to 2 s. A childlike arousal pattern at this age indicates an encephalopathy or a postictal state. Normal Electroencephalogram of Adults The point-form notes preceding the illustrations of normal phenomena adequately describe EEG features of adults. These evolve far less than they do in children.
Normal
Normal Awake:
Pediatrics 3 to 12 months (Figs. 1 to 10)
 Fig 1 Fig 1 |
 Fig 2 Fig 2 |
 Fig 3 Fig 3 |
 Fig 4 Fig 4 |
 Fig 5 Fig 5 |
 Fig 6 Fig 6 |
 Fig 7 Fig 7 |
 Fig 8 Fig 8 |
 Fig 9 Fig 9 |
14 months to 2 years (Figs. 11 to 18)
- Pace of development slows in second year.
- Theta and central rhythms better developed.
- Delta still prominent. Relative paucity of frontal rhythms.
- Eye closure elicits posterior rhythms at higher frequency than in first year.
- Eyes open unless indicated.
 Fig 11 Fig 11 |
 Fig 12 Fig 12 |
 Fig 13 Fig 13 |
 Fig 14 Fig 14 |
 Fig 15 Fig 15 |
 Fig 16 Fig 16 |
 Fig 18 Fig 18 |
3 to 4 years (Figs. 19 to 23, 25 to 27)
- Diffuse theta is prominent;exceeds delta.
- Diffuse delta present.
- Alpha moderately developed when eyes are closed.
- First manifestation of “posterior slow waves” as polyphasic potentials and posterior rhythmic waves.
 Fig 19 Fig 19 |
 Fig 20 Fig 20 |
 Fig 21 Fig 21 |
 Fig 22 Fig 22 |
 Fig 23 Fig 23 |
 Fig 25 Fig 25 |
 Fig 26 Fig 26 |
 Fig 27 Fig 27 |
5 to 10 years (Figs. 24, 28 to 39)
- Slow pace of development.
- Well-developed alpha with eyes closed
- Variable quantity of posterior theta and delta that may be asymmetrical.
- Variable quantity of diffuse theta.
- Delta persists at about 20 to 30µV, principally with eyes open.
- Prominent central rhythm (mu) that may resemble spikes and may be asymmetrical.
 Fig 24 Fig 24 |
 Fig 28 Fig 28 |
 Fig 29 Fig 29 |
 Fig 30 Fig 30 |
 Fig 31 Fig 31 |
 Fig 32 Fig 32 |
 Fig 33 Fig 33 |
 Fig 34 Fig 34 |
 Fig 35 Fig 35 |
 Fig 36 Fig 36 |
 Fig 37 Fig 37 |
 Fig 38 Fig 38 |
 Fig 39 Fig 39 |
11 to 16 years (Figs. 40 to 43, 50)
- Well-developed alpha.
- Variable quantity of posterior slow waves that may be asymmetrical.
- The previous two features in combination create sharply contoured waves that are not spikes.
- Diffuse theta has diminished but continues.
- Minimal diffuse delta, principally with eyes open.
 Fig 40 Fig 40 |
 Fig 41 Fig 41 |
 Fig 42 Fig 42 |
 Fig 43 Fig 43 |
 Fig 50 Fig 50 |
Hyperventilation (Figs. 25, 44 to 49, 51)
- Initially accentuates background including posterior slow waves.
- Posterior build-up usually precedes anterior build-up.
- Asymmetrical bursts normally occur, usually maximum left.
- Sharply contoured waves common.
 Fig 25 Fig 25 |
 Fig 44 Fig 44 |
 Fig 45 Fig 45 |
 Fig 46 Fig 46 |
 Fig 47 Fig 47 |
 Fig 48 Fig 48 |
 Fig 49 Fig 49 |
 Fig 51 Fig 51 |
Normal Drowsiness, Sleep,Arousal: Pediatrics
Drowsy Patterns (Figs. 85 to 92)
Slight increase in ongoing theta and delta in some patients in first year.Spontaneous eye closure may elicit posterior rhythms in early drowsiness, but slower than when awake. Trains of diffuse rhythmic theta may be maximum centrally, posteriorly, or anteriorly. Principally 3 months to 4 years. Most common pattern. Beta accentuation, diffuse or maximal anteriorly or posteriorly, maximal at 5 to 18 months. Decrease in ongoing activity: delta, beta. Combinations of the previous features may occur in sequence: Certain combinations may occur simultaneously.
 Fig 85 Fig 85 |
 Fig 86 Fig 86 |
 Fig 87 Fig 87 |
 Fig 88 Fig 88 |
 Fig 89 Fig 89 |
 Fig 90 Fig 90 |
 Fig 91 Fig 91 |
 Fig 92 Fig 92 |
Burst Drowsy Pattern (Figs. 93 to 96)
Bursts of 2 to 5 Hz sinusoidal waves, usually maximal frontocentrally. Superimposed on other drowsy patterns. Begin at 14 to 18 months; most common at 3 to 5 years; seen until age 11 years.
 Fig 93 Fig 93 |
 Fig 94 Fig 94 |
 Fig 95 Fig 95 |
 Fig 96 Fig 96 |
V Waves (Figs. 97 to 103, 107, 110)
Of higher voltage and briefer than in adults, therefore spike-like. Variable morphology and polarity. May occur sequentially. Shifting asymmetries. Begin at 3 to 4 months, maximal at 3 to 4 years.
 Fig 97 Fig 97 |
 Fig 98 Fig 98 |
 Fig 99 Fig 99 |
 Fig 100 Fig 100 |
 Fig 101 Fig 101 |
 Fig 102 Fig 102 |
 Fig 103 Fig 103 |
 Fig 107 Fig 107 |
 Fig 110 Fig 110 |
Spindles (Figs. 104 to 110, 114, 116)
First clearly expressed at 3 to 4 months. More numerous and longer at 3 to 9 months than later. Asynchrony common in first year. Central–parietal location in early childhood. May be comb-shaped.
 Fig 104 Fig 104 |
 Fig 105 Fig 105 |
 Fig 106 Fig 106 |
 Fig 107 Fig 107 |
 Fig 108 Fig 108 |
 Fig 109 Fig 109 |
 Fig 110 Fig 110 |
 Fig 114 Fig 114 |
 Fig 116 Fig 116 |
V Waves and Spindles (Figs. 107, 110, 115)
V waves, spindles, and other central sleep rhythms combine to create sharply contoured waves that are not spikes.
 Fig 107 Fig 107 |
 Fig 110 Fig 110 |
 Fig 115 Fig 115 |
Positive Occipital Sharp Transients of Sleep (POSTS) (Figs. 147 to 148)
Also known as lambdoid waves. Monophasic. Sharply contoured. Electropositive. Bioccipital. Singly or in 4 to 5 s sequences. Occur in most normal subjects.
 Fig 147 Fig 147 |
 Fig 148 Fig 148 |
OccipitalSharply Contoured Waves and Delta (Figs. 112 to 114)
Normal component of moderate to deep sleep under 5 years.
 Fig 112 Fig 112 |
 Fig 113 Fig 113 |
 Fig 115 Fig 115 |
14 and 6 per Second Positive Spikes (Figs. 117 to 120)
Electropositive sharp components repeat at 14 and/or 6 to 7 Hz per second.
Positive component apiculate or arciform. Negative component smooth. Occur singly or in bursts. 13 to 17 Hz or 6 to 7 Hz; principally 14 or 6 Hz. Posterior temporal and adjacent areas. Widespread field. Best recorded with coronal or referential montages. Duration: Seen in adolescents and young adults. Occur during drowsiness and sleep.
Normal.
 Fig 117 Fig 117 |
 Fig 118 Fig 118 |
 Fig 119 Fig 119 |
 Fig 120 Fig 120 |
Arousal Sequence (Figs. 121 to 124)
Initial stimulus evokes one or more broad V waves. Then 4 to 8 Hz diffuse rhythmic waves, maximum frontal-central occasionally mixed with delta.
Then 1 to 3 Hz diffuse delta. Posterior delta is independent of anterior delta and persists longer. Then delta merges with 4 to 5 Hz waves.
 Fig 121 Fig 121 |
 Fig 122 Fig 122 |
 Fig 122 Fig 122 |
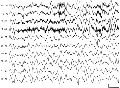 Fig 124 Fig 124 |
References
- Aird RB, Gastaut Y. Occipital and posterior electroencephalographic rhythms. Electroencephalogr Clin Neurophysiol. 1959;11:637–656.
- Berger H. On the electroencephalogram of man. Fifth report. In: Gloor P, ed. Electroencephalogr Clin Neurophysiol (suppl 28). Amsterdam: Elsevier; 1969:151–171.
- Brandt S, Brandt H. The electroencephalographic patterns in young healthy children from 0 to five years of age. Acta Psychiatr Neurol Scand. 1955;30:77–89.
- Corbin HPF, Bickford RG. Studies of the electroencephalogram of normal children: comparison of visual and automatic frequency analyses. Electroencephalogr Clin Neurophysiol. 1955;7:15–28.
- Cornil L, Gastaut H. Donnees electroencephalographiques sur la dominance hemispherique. Rev Neurol. 1947;79:207.
- Dale PW, Busse EW. An elaboration of a distinctive EEG pattern found during drowsy states in children. Dis Nerv Syst. 1951;12:122–125.
- Demerdash A, Eeg-Olofsson O, Petersen I. The incidence of 14 and 6 per second positive spikes in a population of normal children. Dev Med Child Neurol. 1968;l0:309–316.
- Dreyfus-Brisac C. The EEG during the first year of life. In: Lairy GC, ed. Handbook of Electroencephalography and Clinical Neurophysiology, vol 6B: The Evolution of the EEG from Birth to Adulthood. Amsterdam: Elsevier; 1975;25–30.
- Drury I. 14 and 6 Hz positive bursts in childhood encephalopathies. Electroencephalogr Clin Neurophysiol. 1989;72:479–485.
- Eeg-Olofsson O. The development of the electroencephalogram in normal children from the age of 1 through 15 years. 14 and 6 Hz positive spike phenomenon. Neuropadiatrie. 1971;2:405–426.
- Fisch BJ. Fisch and Spehlmann's EEG Primer. Basic Principles of Digital and Analog EEG. 3rd ed. Amsterdam: Elsevier Science, 1999. Gibbs FA, Gibbs EL. Atlas of Electroencephalography. Vol 3. Neurological and Psychiatric Disorders. Reading, PA: Addison Wesley; 1964:136.
- Goodwin JE. The significance of alpha variants in the EEG and their relationship to an epileptiform syndrome. Am J Psychiatry. 1947:369–379.
- Hagne I. Development of the waking EEG in normal infants during the first year of life. In: Kellaway P, Petersen I, eds. Clinical Electroencephalography of Children. New York: Grune & Stratton; 1968:97–118.
- Hagne I, Persson J, Magnusson R, Petersen I. Spectral analysis via Fast Fourier transform of waking EEG in normal infants. In: Kellaway P, Petersen I, eds. Automation of Clinical Electroencephalography. New York: Raven Press; 1973:103–143.
- Kellaway P. An orderly approach to visual analysis: Characteristics of the normal EEG of adults and children. In: Daly DD, Pedley TA, eds. Current Practice of Clinical Electroencephalography. New York: Raven Press, 1990:139–199.
- Kellaway P, Fox BJ. Electroencephalographic diagnosis of cerebral pathology in infants during sleep. J Pediatr. 1952;41:262–287.
- Kooi KA, Tucker RP, Marshall RE. Fundamentals of Electroencephalography. 2nd ed. Hagerstown, MD: Harper & Row; 1978:68.
- Lenard HG. The development of sleep spindles in the EEG during the first two years of life. Neuropaediatrie. 1970;1:264–276.
- Lombroso CT, Schwartz IH, Clark DM, Muench H, Barry J. Ctenoids in healthy youths. Neurology. 1966;16:1152–1158.
- Metcalf DR. Controlled studies of the incidence and significance of 6 and 14 per sec positive spiking. Electroencephalogr Clin Neurophysiol. 1963;15:161(P).
- Pampiglione G. Some criteria of maturation in the EEG of children up to the age of 3 years. Electroencephalogr Clin Neurophysiol. 1972;32:463(P).
- Perez-Borja C, Chatrian GE, Tyce FA, Rivers MH. Electrographic patterns of the occipital lobe in man. Electroencephalogr Clin Neurophysiol. 1962;14:171–182.
- Petersen I, Akesson HO. EEG studies of siblings of children showing 14 and 6 per second positive spikes. Acta Genet (Basel). 1968;l8:163–169.
- Petersen I, Eeg-Olofsson O. The development of the electroencephalogram in normal children from the age of 1 through 15 years. Nonparoxysmal activity. Neuropadiatrie. 1971;2:247–304.
- Pond DA. The development of normal rhythms. In: Hill C, Parr G, eds. Electroencephalography. 2nd ed. London: Macdonald, 1963:193–206.
- Reiher J, Carmant L. Clinical correlates and electroencephalographic characteristics of two additional patterns related to 14 and 6 per second positive spikes. Can J Neurol Sci. 1991;18:488–491.
- Samson-Dollfus S, Goldberg P. Electroencephalographic quantification by time domain analysis in normal 7–15-year-old children. Electroenc ephalogr Clin Neurophysiol. 1979;46:147–154.
- Schulte FJ, Bell EF. Bioclectric brain development. An atlas of EEG power spectra in infants and young children. Neuropadiatrie. 1973;4:30–35.
- Schwartz IH, Lombroso CT. 14 & 6/second positive spiking (ctenoids) in the electroencephalograms of primary school pupils. J Pediatr. 1968;72:678–682.
- Silverman D. Phantom spike-waves and the fourteen and six per second positive spike pattern. Electroencelphalogr Clin Neurophysiol. 1967;23:207–213.
- Slater GE, Torres F. Frequency–amplitude gradient. A new parameter for interpreting pediatric sleep EEGs. Arch Neurol. 1979;36:465–470.
- Tanguay PE, Ornitz EM, Kaplan A, Bozzo ES. Evolution of sleep spindles in childhood. Electroencephalogr Clin Neurophysiol. 1975;38:175–181.
- Westmoreland BF, Sharbrough FW. Posterior slow wave transients associated with eye blinks in children. Am JEEG Technol. 1975;15:14–19.
Source:
